
Get patients started on the only once-daily pill approved for bronchiectasis1
Ways to prescribe
Initiate patients on BRINSUPRI using the BRINSUPRI Prescription and inLighten™ Enrollment Form.
When completing the form:
Fill out all required fields in the prescription section, including:
Number of refills
Your signature
Remember to collect both patient signatures in the enrollment section—one for consent and the other to authorize access to support services
Include key information on your patient’s pharmacy benefit plan (including BIN, PCN, and group number) to help streamline the administrative process
or
E-prescribe via an EHR. BRINSUPRI is a limited-distribution product and can only be dispensed bya:
Amber Specialty Pharmacy: (888-370-1724) (NCPDP: 2815338)
Maxor Specialty Pharmacy, A VytlOne Company: (800-658-6046) (NCPDP: 4575518)
PANTHERx Rare Pharmacy: (855-726-8479) (NCPDP: 6008002)
Patients who are e-prescribed can enroll separately to receive patient support from inLighten by calling 833-LIGHT-00 (833-544-4800) or visiting brinsupri.inlightensupport.com.
Prescribe BRINSUPRI and you can help patients enroll in the inLighten Patient Support program



Patients can receive support from inLighten by:
Completing and signing the enrollment form in-office
Enrolling at brinsupri.inlightensupport.com
Calling 833-LIGHT-00 (833-544-4800) Monday through Friday, from 8 AM to 8 PM ET and speaking with an inLighten Coordinator
You can help patients already prescribed BRINSUPRI sign up for the support program by completing the inLighten enrollment form with them.
Get the BRINSUPRI Prescription and inLighten Enrollment Form.
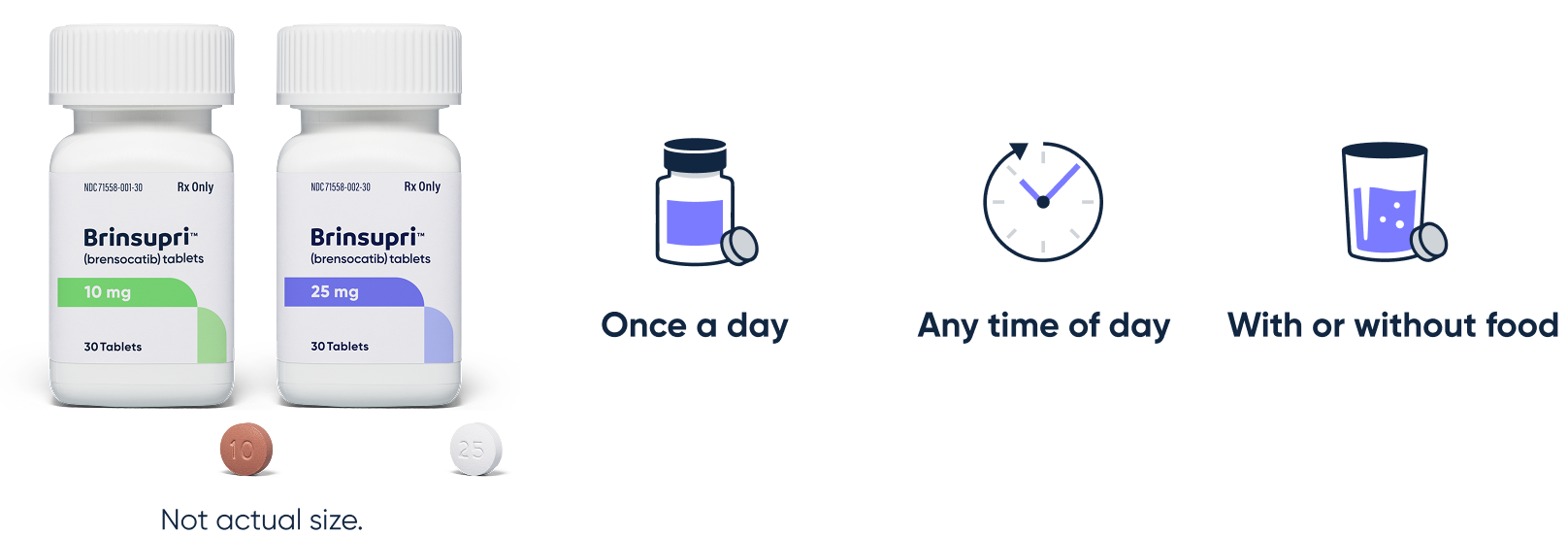
BRINSUPRI is one pill, once a day1
BRINSUPRI may fit into a multimodal approach to managing bronchiectasis and can be taken:

Dosing
BRINSUPRI is available in dosage strengths of 10 mg or 25 mg to be taken orally once daily.
Remind patients that results can take time and may vary, and they should speak with their doctor before making any changes in treatment. If a patient misses a dose, they should take the next dose at their regular time the next day. They should not double the dose to make up for the missed dose.
